Contact-dependent growth inhibition (CDI)
The first soluble bactericidal factor was described by André Gratia in 1925. Shortly thereafter, Alexander Fleming discovered penicillin, which is a β-lactam antibiotic released by Penicillium fungi to kill competing bacteria. Although diffusible antibacterial compounds have been known for a century, more recent research shows that bacteria commonly deliver toxic effector proteins directly into neighboring competitors. In 2005, David Low's group at UC Santa Barbara identified a novel mechanism of inter-bacterial competition that requires direct cell-to-cell contact. Contact-dependent growth inhibition (CDI) is a function of the CdiB and CdiA two-partner secretion (TPS) proteins. CDI systems assemble toxic CdiA filaments that project several hundred angstroms from the cell surface. Upon binding specific receptors on neighboring bacteria, CdiA undergoes a series of conformational changes that ultimately deliver the C-terminal toxin (CdiA-CT) domain into the target cell. The animation in Fig. 1 outlines our current mechanistic understanding of these remarkable systems.
Polymorphic CDI toxin-immunity protein pairs
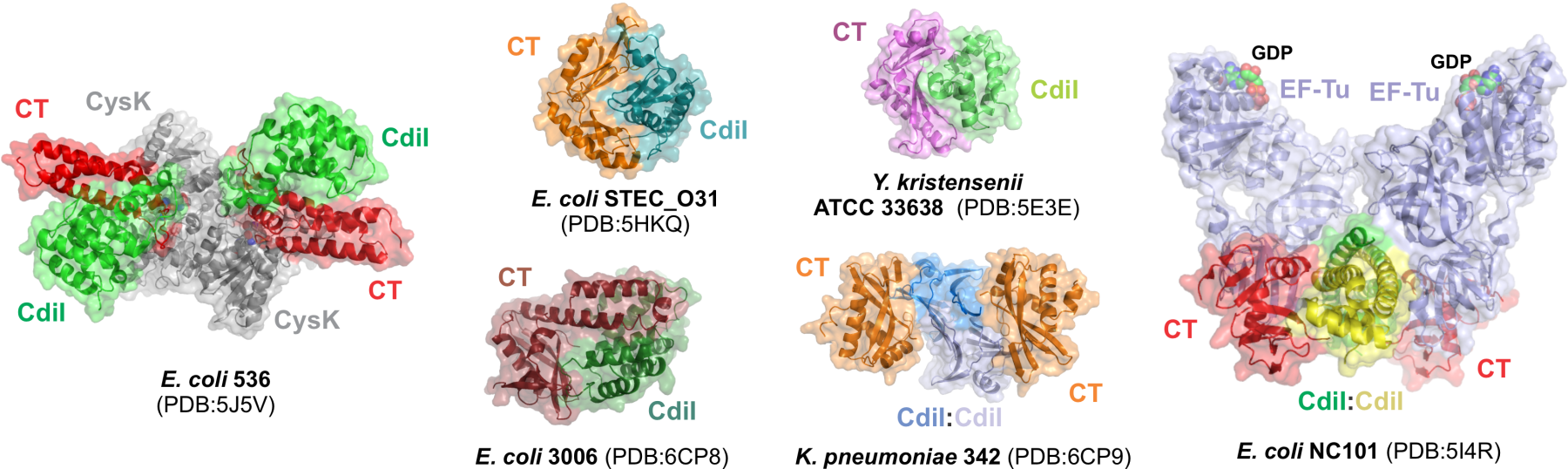
CdiA effector proteins are characterized by the extraordinary diversity of C-terminal toxin domains that they deploy. A survey of CdiA-CT toxins found in all E. coli isolates reveals 21 distinct toxin types. In collaboration with Celia Goulding (UC Irvine) and Karolina Michalska (Argonne National Lab), we have determined the structures of several CDI toxins and CdiI immunity proteins (Fig. 2) and characterized their biochemical activities. Most of these toxins are ribonucleases with unusual substrate specificities. CDI toxins also contain highly variable "cytoplasm entry" domains, which are required for toxin translocation across target-cell membranes. How the various entry domains hijack integral membrane proteins to mediate toxin import remains an active area of research.
Fig. 2. Crystal structures of CdiA-CT•CdiI complexes. Structures of CDI toxin•immunity protein complexes from various enterobacterial species. In some instances (E. coli 536 and NC101), these proteins form ternary complexes with accessory proteins like CysK and EF-Tu.
The Type VI Secretion System (T6SS)
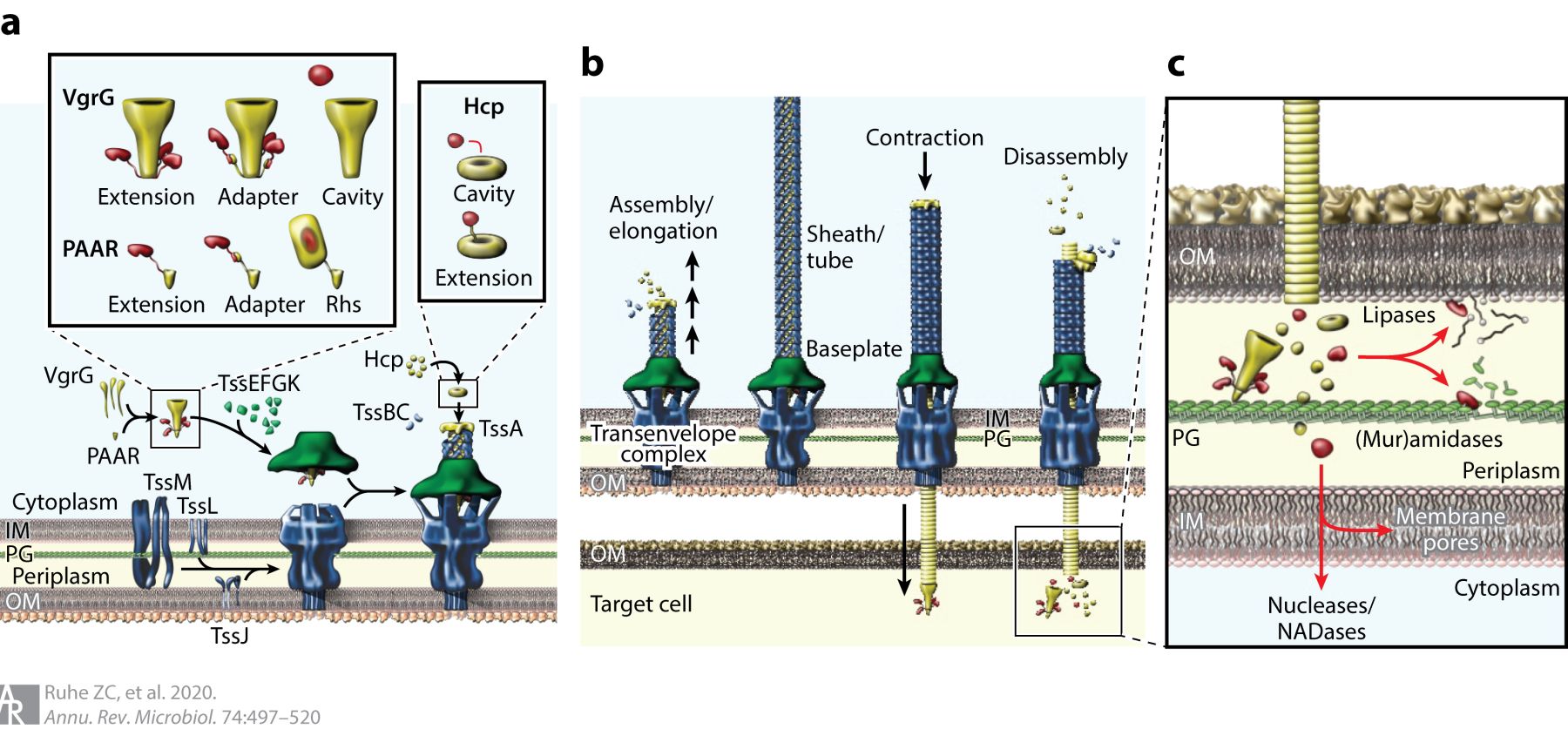
After the discovery of CDI, the research groups of Joseph Mougous and Stefan Pukatzki showed independently that type VI secretion systems (T6SS) mediate contact-dependent competition. The T6SS apparatus is a multi-protein complex that functions like a contractile bacteriophage to eject a toxin-laden projectile into neighboring bacteria (Fig. 3). We were the first to show that Rearrangement hot-spot (Rhs) proteins are toxic effectors deployed by T6SSs. Like CdiA proteins, Rhs effectors are large, peptide-repeat proteins that carry polymorphic C-terminal toxin domains. However, Rhs repeats form a β-cocoon structure that encapsulates the toxin domain. Characterized Rhs-CT domains have DNase, NADase, ADP-ribosylase and ionophoric activities, and these toxins are some of the most potent T6SS effectors known. We are interested in the mechanisms by which Rhs effectors – and distantly related wall-associated protein (WapA) toxins – deliver their encapsulated payloads into target bacteria.
Fig. 3. Type VI secretion systems. (a) T6SS subcomplex assembly and effector packaging onto VgrG and Hcp. (b) Sheath-tube assembly initiates at the baseplate and extends across the width of the cell. The sheath rapidly contracts, expelling the Hcp tube through the baseplate and transenvelope complexes. The PAAR-tipped VgrG protein penetrates the outer membrane of the target bacterium and releases effector proteins. (c) T6SS effector protein activities. Nucleases and NADases are translocated into the cytoplasm through poorly understood pathways. Abbreviations: Hcp, hemolysin-coregulated protein; IM, inner membrane; NADase, NAD(P)+ glycohydrolase; OM, outer membrane; PG, peptidoglycan; Rhs, rearrangement hotspot; T6SS, type VI secretion system; VgrG, valine-glycine repeat G protein.